The “Glass Earth” – Geochemical Frontiers in Exploration Through Cover
Graham Carr1, Anita Andrew2, Geoff
Denton1, Angela Giblin1, Michael Korsch1and
David Whitford2
1. CSIRO Exploration and Mining
2. CSIRO Petroleum Resources
Key Words: Glass Earth, geochemistry, groundwater, selective leach, isotopes, data integration
Introduction
“Glass Earth” represents a number of current and planned projects within CSIRO aimed at making “transparent” the top 1000 m of the Earth’s crust. It builds upon current technologies developed within a number of CSIRO divisions as well as the Australian Mineral Exploration Technologies CRC (AMET CRC), the Australian Geodynamics CRC (AG CRC) and the CRC for Landscape Evolution and Mineral Exploration (CRC LEME). The following new geophysical and geochemical technologies will be developed to complement these, together with new capabilities in modelling, data integration and visualisation.
· tensor gradient magnetometry
· gravity gradiometry
· airborne chemical mapping
· hydrogeochemistry, hydrogeology, surface geochemistry and isotope geochemistry
· modelling of chemical, fluid and heat flows in rock and regolith
· advanced visualistion and data fusion.
This paper will describe some recent work in the field of geochemistry, with the principal aim of “seeing through” cover to understand basement geology and detect hidden ore systems.
The problems
With some exceptions (e.g. Lintern and Butt, 1993) geochemistry is not a well-validated technique for exploration through cover. The problem is that the majority of media sampled in the near-surface environment are part of the transported sequence of sediments, sedimentary rocks or more recent volcanic rocks that cover the older, prospective ore-bearing units. Unless there has been some geochemical connection between the underlying and cover rocks, there can be no hope of detection via geochemistry. There are two mechanisms that could possibly provide such a nexus.
· Groundwater is an active medium in the regolith and underlying bedrock and can transport metals both laterally and vertically. That such movement exists, is readily exemplified in a gross sense by the problems of soil salination and the formation of acid-sulfate soils in response to rising water tables. In the former case, the salinity results from the gradual uptake of conservative ions (principally Na and Cl) from “old” groundwater that has moved great distances through aquifer rocks. Acid sulfate soils, however, are likely to result from more local transport and deposition of non-conservative ions such as SO4 and Fe. Other more specific examples will be given below. Ore and indicator elements liberated into groundwater solution could be transported substantial distances laterally before they reached the water table.
· Another potential mechanism of transport is via a gas phase. There are well-documented examples of gases moving from considerable depth to the surface, for example volatile organic compounds related to deep hydrocarbon sources, inert gases and Hg (Carr et al., 1986). In addition, there is evidence for an ascending microflow of “geogas” (Malmqvist and Kristiansson, 1984). It has been speculated that such a gas could be a carrier for the movement of other elements, including metals, in simple or complex forms (Kristiansson et al., 1990, Johnson et al., 1994, Wang et al., 1999). Metals transported in such a gas phase would move principally vertically. The application of selective leach techniques is built on the supposition that many metals can move from the hypogene zone (down to about 1000 m) through the regolith to collect in surface soils. Preferential leaching of specific soil components using various standard and proprietary reagents is believed to identify these gas-transported metals. While some case history studies appear to vindicate this hypothesis (e.g Mann et al., 1993), anecdotal evidence of many exploration applications of the technique provide equivocal results. Such results are to be expected, considering the uncertainties as to the mechanism of metal transport and formation of selective leach anomalies.
Applications of Groundwater Chemistry
There are three fundamental questions that need to be asked when assessing the potential application of groundwater techniques in specific terrains.
1. Can basement geology be characterised through the analysis of groundwater within overlying sequences?
2. Can groundwater chemistry indicate the presence of mineralisation in basement rocks?
3. What is the scale of dispersion of mineralisation and indicator elements from basement into aquifers in the overlying sequences?
Such questions need to be asked and answered in relation to individual geological and geomorphic terrain types. To do this we need to understand the interaction of groundwater and aquifer rocks and thus the processes of mineral dissolution and precipitation, and transport of the major and trace element components of groundwater
Assessment of bedrock geochemistry. Giblin (1998) has undertaken a regional study of groundwater chemistry within the Carpentaria and Eromanga Basins where the underlying basement rocks of the Georgetown and Mt Isa Blocks are potential hosts of major base metal ore systems, such as Cannington and Dugald River. Results suggest that variations in major element chemistry of groundwater sampled from near the top of the water table are an indicator of depth to basement. The major ions in waters from within basement rocks are characterised by high Mg, Ca and SO4 and contrast with waters within the deepest parts of the basin cover sequences that have higher Na and HCO3. In Figure 1, scores based on a Principal Component analysis of major elements show the regional distribution of these contrasting water types and suggest a relationship between water chemistry and depth to basement. Giblin (1998) interprets this to be a result of mixing of waters that have penetrated basement with waters sourced wholly within the cover sequence. Another possibility is that the differences relate to variations in mineral chemistry between different aquifers in the cover sequences, which is dependent in some way on basin thickness.
Indications of mineralisation There are some examples in the literature showing a geochemical fingerprint of mineralisation in groundwater (e.g. Giblin 1997, Giblin and Mazzuchelli, 1997). We present here the example of the Abra deposit in Western Australia (Whitford et al., 1998). Mineralisation at Abra consists of a Pb-Zn stringer zone enriched in Cu, Au and barite. The deposit is blind and is overlain by 200 m of Proterozoic meta-sediments. Mineralisation can be detected in groundwater from both major and trace elements and isotopes. Indicators include Pb isotope signatures, relatively high d34S values, enrichments in Ba, Cs, As, Cu and Mn and high 87Sr/86Sr ratios which probably reflect an alteration envelope (Fig. 2).
Scale of Dispersion There is a dearth of good data that can give clues to the likely scales of dispersion of elements in groundwater around a blind ore deposit. Based on a series of case histories, involving a combined multi-element and isotope approach to groundwater analysis, Carr et al. (1994) concluded that dispersion of between tens of metres and several kilometres were likely for the elements Pb, Sr and S. The distribution of d34S around the Menninnie Dam deposit on the Gawler Platform in shown in Figure 3 and indicates dispersion of over 1 km from the blind sulfide mineralisation (Andrew et al., 1998).
Application of Surface Geochemistry (Selective Leach) Techniques
We neither wish, nor are able to comment on the relative merits of different selective leaches. The current interest in this technology is based on a large number of case history examples that seem to point to a relationship between the concentration of the most labile metal component in surface soils and the presence of a buried or blind mineralisation at some depth below the surface. However, there is an element of cynicism in the geochemical fraternity that goes beyond a reaction to some of the more extravagant claims of the geochemical analytical industry, but relates more to the uncertainty of how to interpret selective leach results, their reproducibility and their ability to discriminate between significant and insignificant targets. These uncertainties will not be allayed until there is a much better understanding of the processes involved in their formation.
We can speculate on four possible mechanisms by which such anomalies could form.
1. Vertical migration of metals from an underlying source in a gas carrier phase (“geogas”) or due to an electro-potential.
2. Interaction of geogas and soil, or a more local in-soil effect, resulting in the dissolution of metals within the soil profile.
3. Interaction of soils and rising groundwater, or groundwater vapour - i.e a proxy for a groundwater geochemical anomaly.
4. Local and essentially residual anomalies that result from dissolution of metal-rich minerals within the soil profile or immediately underlying rocks.
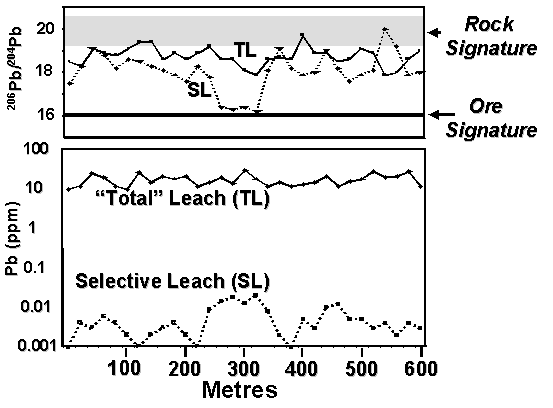
Although difficult to reconcile with mainstream inorganic and physical chemistry as a feasible process for metal transport, it is only the first of these possibilities that is generally considered in the interpretation of selective leach data. However, one or all of these mechanisms may apply in many environments. Isotope fingerprinting has the potential to discriminate the likely source of an anomaly. Both the Pb and the S isotope systems can identify Mechanism 2. Isotope signatures, combined with orientation groundwater chemistry and soil profile geochemistry, will discriminate all four mechanisms. A hypothetical soil traverse across a buried ore system is given in Figure 4 based on Pb isotope systematics. In this case, the isotopic signature of Pb derived from ore is distinctly different from Pb derived by dissolution of Pb from within the soil profile (“Rock Signature”). The selective leach anomaly at position 300 m is related to an ore-Pb source, whether through Mechanisms 1, 3 or 4. In contrast, the selective leach anomaly at position 450 m is unrelated to an ore-Pb source and results from Mechanism 2. Some preliminary examples of the Pb isotope analysis of selective leach solutions, undertaken at CSIRO, are consistent with this hypothetical. One is presented in Figure 5.
Data Integration and Analysis
The use of the above geochemical techniques in the search for mineralisation beneath cover needs to be in the context of a comprehensive and well planned exploration strategy. Considered in isolation of other data inputs, such as mapping of both cover and outcrop geology and geophysical assessment of basin morphology and ore targets, such geochemistry can be at best uninterpretable and at worst misleading. What is required is the parallel development of technologies that allow integration of all data and the assessment of often diverse interpretations of such data to achieve the “most likely” inversion to basin and basement geology.
Conclusions
Groundwater provides a mechanism for transporting ore-associated elements from a deeply buried source to the near surface where they are relatively easily sampled. We believe groundwater chemistry has the potential to be a powerful technology in locating buried and blind ore systems. Further research and development is required to;
· understand dispersion around ore systems and interaction with aquifer rocks,
· optimise groundwater sampling and analytical techniques to reduce costs and increase reliability, and
· develop a database of Australian groundwater data and accessible interpretation techniques.
The use of selective leach technologies also has potential in assessing the prospectivity of buried terrains, however there is no clear understanding of the processes of formation of selective leach anomalies and thus how to interpret them in the wide variety of geologic, geomorphic and climatic environments of Australia. Research must concentrate on understanding mechanisms and verifying these in comprehensive case history studies.
In both fields, modern advances in isotope geochemistry will prove crucial. In particular, the ability to undertake high precision, low cost analyses using the modern generation multi-collector ICPMS (for heavy isotopes) and continuous flow IRMS (for light isotopes) will not just provide tools to understand the processes, but will also provide an accessible technology for the discrimination of “anomalous” geological materials.
References
Andrew, A.S., Carr, G. R., Giblin, A.M. and Whitford, D.J., 1998. Isotope hydrogeochemistry in exploration for buried and blind mineralisation. In, Eggleton, R.A. (Ed), The State of the Regolith. Geol. Soc. Aust. Special Publication No 20, pp. 220 – 225.
Carr, G.R., Wilmshurst, J.R. and Ryall, W.R., 1986. Evaluation of mercury pathfinder techniques: base metal and U deposits. J. Geochem. Explor., 26, pp. 1-117
Carr, G.R., Whitford, D.J., Andrew, A.S., and Giblin, A.M.,1994. Exploration for concealed mineralization: Multi-isotopic studies of groundwaters. Final Report. AMIRA Project P338. CSIRO Division of Exploration and Mining Restricted Report 15R, 33p.
Giblin, A., 1996. An application of groundwater
geochemistry to the detection of prospective basement beneath Mesozoic cover in
North Queensland. In, Mesozoic
Geology of the Eastern Australia Plate, Geol.
Soc. Aust. Ext. Abstr., 43, pp. 186-194.
Giblin, A.,
1997. Geochemistry of groundwaters in the vicinity of Stawell, Clunes, Ararat
and Ballarat gold deposits. In Anon,
The AusIMM 1997 Annual Conference,
Ballarat, 12-15 March, 1997 Publ. Ser. 1/97, pp.181-191.
Giblin, A.
and Mazzucchelli, R., 1997. Groundwater geochemistry in exploration: an
investigation in the Black Flag district, Western Australia. Aust. J. Earth Sci., 7, 44, pp. 433-443.
Johnson, C., Rutherford, N.F. and Giblin, A.M., 1994. Sirogas AMIRA Project 381(extension) Final Report.CSIRO-RIR 450R, 105p.
Kristiansson, K. , Malmqvist, L. and Persson, W., 1990. Geogas prospecting: a new tool in the search for concealed mineralisations. Endeavour, New Series, V14, No 1, pp. 28 – 33.
Lintern, M., and Butt, C., 1993. Pedogenic carbonate: an important sampling medium fro gold exploration in semi-arid areas. Explor. Res. News, 7, pp. 7, 10 – 11.
Malmqvist, L. and Kristiansson, K., 1984. Experimental evidence for an ascending microflow of geogas in the ground. Earth Planet. Sci. Lett., 70, pp. 407 – 416.
Mann, A.W., Birrell, R.D., Lawrance, L.M., Mann, A.T. and Gardner, K.R., 1993. The new mobile metal ion approach to the determination of buried mineralisation. In, Williams, P.R., and Haldane, J.A., (Compilers), Kalgoorlie 93. An International Conference on Crustal Evolution, Metallogeny and Exploration of the Eastern Goldfields. Extended Abstracts, Australian Geological Survey Organisation, Record 1993/54, pp. 223-228.
Wang, X. Xie, X, Cheng, Z and Liu, D, 1999. Delineation of regional geochemical anomalies penetrating through thich cover in concealed terrains – a case histroy from the Olympic Dam deposit, Australia. In, Bogoch, R. and Shirav, M., (eds) Geochemical Exploration 1997, J. Geochem. Explor., v66, Nos 1 – 2, pp. 85 –113.
Waring, C., Andrew, A.S. and Ewers, G.R., 1998. Use of O, C, and S stable isotopes in regional mineral exploration. AGSO Jour. Aust. Geol. Geophys., 17(4), pp. 301 – 313.
Whitford, D.J., Andrew, A.S., Carr, G.R. and Giblin, , A.M., 1998. Application of isotope studies of Australian groundwaters to mineral exploration: The Abra Prospect, Western Australia. In Arehart , G. B, and Hulston, J.R., (Eds), Proceedings of the 9th International Symposium on Water-Rock Interaction, pp. 583-586.
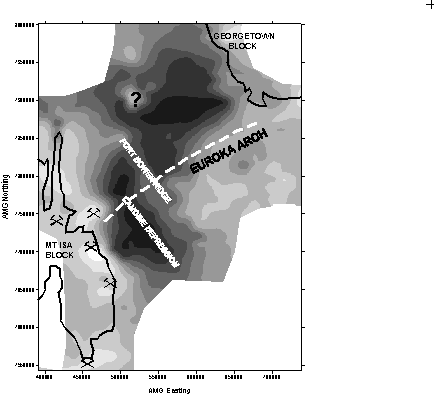
Figure 1. Contoured
map showing Principal Component scores for groundwater within the
Carpentaria and Eromanga Basins and adjacent basement terrains. Note the
close relationship between scores and basin structure (From Giblin, 1996).
Figure 2.
Contour maps showing the distribution of S, Pb and Sr isotopic composition
in groundwater around the Abra Prospect.
The heavy line represents the surface projection of the mineralized
zone (After Whitford et at., 1998)
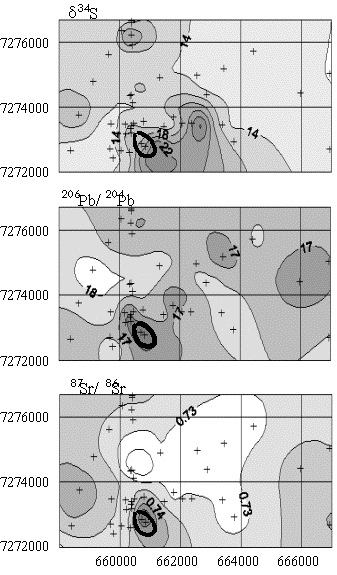
Figure 3 Plan maps about the Menninnie Dam
deposit in South Australia showing distribution of S isotope values. Regional background d34S values are in the range 16 – 20 ‰ (CDT)and S from the
mineralisation hosted in the Hutchison Group and buried Gawler Range
Volcanic cover has a value of 0 – 5‰ (CTD). Groundwaters within and down
slope (to the south) from the deposit show evidence of mixing between
these two sources (After Waring et al., 1998).
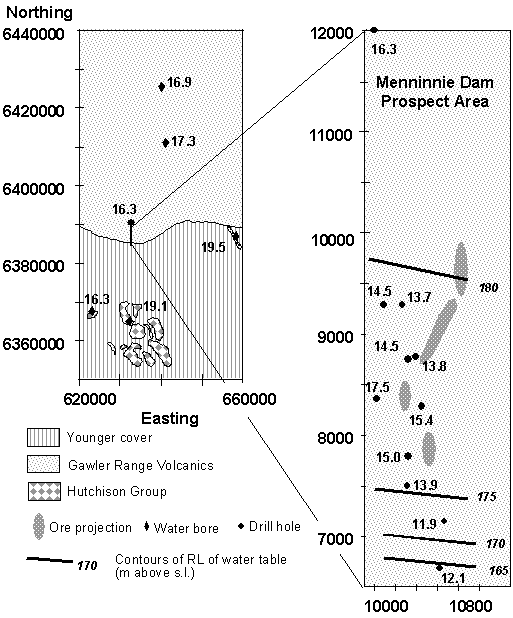
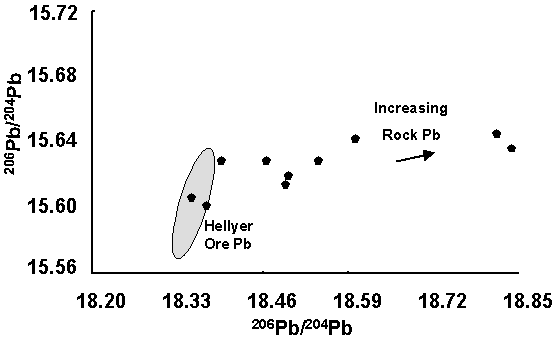
Figure 4 A hypothetical soil traverse comparing
Pb concentration and Pb isotope ratios of selective and total
leaches. Selective leach anomalies
derived from ore-Pb will have a characteristic isotopic signature (350 m) significantly different to Pb
derived from unmineralised rock
(e.g 450 m). Figure 5 Results of Pb isotope analysis of selective leaches of some
Western Tasmanian soils. Samples with the Hellyer ore-Pb signature
contained anomalous Selective Leach Pb and those with a rock-lead
signature had background Selective Leach Pb.